The basics of Carbon 14
The basics of Carbon 14
Radiocarbon dating has been one of scientific advances which has had the greatest impact to the study of the past. We normally associate this technique with archaeology but applications have also been found in the fields of geology, hydrology, geophysics, atmospheric sciences, oceanography, palaeoclimatology, and even biomedicine.
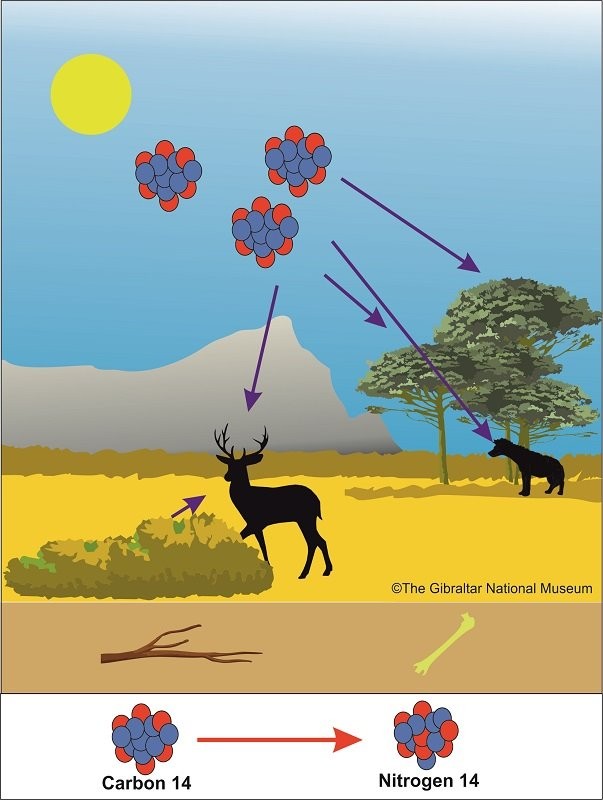
Radiocarbon, or carbon 14 (14C), is an unstable and slightly radioactive isotope of the element carbon. The stable isotopes are carbon 12 and carbon 13. Plants and animals assimilate Carbon 14 from carbon dioxide in the atmosphere throughout their lives. Once they die, they stop exchanging carbon with the biosphere and their carbon 14 content begins to decrease as it transforms into Nitrogen 14 at a particular rate, and by knowing the annual loss rate of carbon 14, we can determine the date that a particular organism had died and had stopped assimilating carbon 14.
The kinds of materials that can be dated using this technique include charcoal, wood, seeds, bones, leather, peat, clay, lake mud, soil, hair, pottery, pollen, murals, corals, blood residues, fabrics, paper or parchment, resins and water.
The main limitation of radiocarbon dating is the maximum, or oldest, age that can be measured. This age limit is 45,000-50,000 years, which is the time it takes for all the carbon 14 in a sample to decay.
There are three main techniques used to measure the carbon 14 content of a sample: gas proportional counting, liquid scintillation counting and accelerator mass spectrometry.
This dating technique has been applied at various sites around Gibraltar: Gorham’s Cave, Ibex Cave, Devil’s Tower Rock Shelter, Bray’s Cave and Vanguard Cave. In this last cave, due to practically its entire archaeological sequence being older than 50,000 years, other dating techniques have had to be employed.
Published: May 04, 2020
Other similar Virtual Museum
Other similar VM - Methodology
Virtual Museum VM - Historical Notes from our Archive
historical notes from our archive a. e. serfaty
Published: April 04, 2020
Virtual Museum VM - Historical Notes from our Archive
Historical Notes from our Archive The Paint Supply Co.
Published: April 16, 2020
Virtual Museum VM - Historical Notes from our Archive
Historical Notes from our Archive The Rock Hotel
Published: April 20, 2020
18-20 Bomb House Lane
PO Box 939,
Gibraltar
